Technology
Principles of Fuel Cells
Fuel cells convert chemical energy directly into electrical energy and heat. In this respect, fuel cells are comparable to batteries. However in contrast to batteries or accumulators, the chemical reactants are supplied continuously. This makes it interesting for durable operation in many applications. Due to its principle, fuel cells feature numerous advantages, which make them
attractive for new products and sustainable supply structures:
- High electrical efficiency (from full to part load)
- Low noise emissions, as few moving parts (only in peripheral devices) are necessary
- Up to 50% lower ratio of CO2 emissions compared with conventional power stations
- Almost no emissions of pollutants like NOX, SO2, and CO
Fuel Cell Types
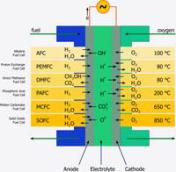
A large diversity of fuel cell types exist that differ in the kind of the electrolyte and the operation temperature. So the right cell can be found for every application. Numerous materials and shapes support the electrochemical process, which is going on in fuel cells. All cells do have one common characteristic: a conductive electrolyte that separates anode and cathode and allows only ions (as charge carriers) to pass. The electrons that are required for the chemical reaction have to move via the external electrical circuit - providing electrical power. The driving force is an oxygen partial pressure difference between cathode air and anode fuel. This mentioned principle underlies all fuel cells known until now.
Fuel cells are distinguished in according to its type of electrolyte material. This can be liquid or solid, it can comprise leach, polymer, salt or ceramic and determines the operating temperature. Relating to the material, temperatures vary from ambient up to 1000 °C that usually define the typical application fields.
SOFC – Solid Oxide Fuel Cell
As the name SOFC implies, all components of this fuel cell (anode, cathode, electrolyte and interconnect) are solid and became a big research topic since the 1950s. The use of ceramics allows the fabrication of different designs like tubular or planar cells. The SOFC offers great advantages like the high operating temperature which allows internal reforming, fuel flexibility and the decoupling of the by-product process heat. Due to the fact that oxygen ions are the charge carriers, H2 and CO can be used as fuels. Simple pre-reformers, regardless of the technology (ATR, CPOX or SR), can provide them from different hydrocarbons. Cause of this fuel independence, the SOFC is the outstanding power plant technology now and in the future.